To reduce the corrosion of metals, various methods can be used:
- Electrolytic surface treatment, electroplating (galvanizing, zinc-nickel electroplating, etc.)
- A choice of suitable materials
- A design of the product
- Proper installation and maintenance
Corrosion process
Chemical and electrochemical corrosion
Chemical corrosion can be considered as oxidation and occurs through the action of dry gases, often at high temperatures. Electrochemical corrosion, on the other hand, occurs through electrode reactions, often in humid environments, ie wet corrosion. All metals in dry air are covered by a very thin layer of oxide, about 100Å (10-20nm) thick. This layer is made up of a chemical reaction with the oxygen in the air. At very high temperatures, the reaction with the oxygen in the air can continue without restraint and the metal will quickly transform into an oxide.
The corrosion of steel can be considered as an electrochemical process that takes place in several steps. The initial attack occurs at anodic sites on the surface where iron ions dissolve. Electrons are released from the anode and move through the metal structure to the adjacent cathodic sides of the surface, where they combine with oxygen and water to form hydroxyl ions. Furthermore, hydroxyl ions react with the iron ions from the anode to form iron hydroxide, which itself is further oxidized in air to produce hydrogenated iron oxide (ie red rust). The sum of these reactions can be represented by the following equation:
Wet time is the proportion of the total time during which the surface is wet due to rain or condensation. It follows that for unprotected steel in dry environments, e.g. indoors, corrosion will be minimal due to low availability of water.
The driving force for the current flow and thereby the corrosion is the difference in the electrode potential. The electrode potential of a metal is an indication of the tendency of the metal to dissolve and corrode in a particular electrolyte. The nobler the metal, the higher the potential, the less it tends to dissolve in an electrolyte.
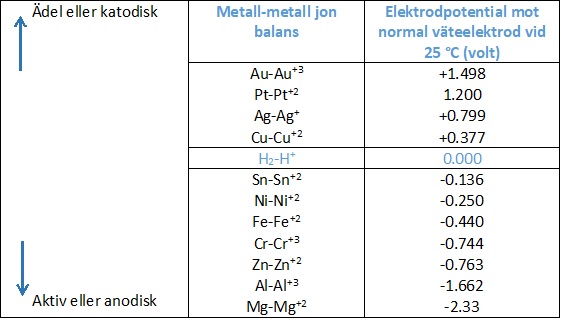
With regard to the iron-copper example, it will be noted from the table above that copper has a higher potential (is nobler) than iron. The iron will be the anode and corrode, while the copper will be the cathode and not corrode.
Different types of corrosion
General corrosion
General corrosion is characterized by an overall attack on the surface. Corrosion occurs without distinct anodic and cathodic areas.
Local corrosion
Local corrosion is the opposite of general corrosion. In the case of local corrosion, most of the metal surface is unaffected and only small areas are strongly affected. It is much easier to compensate for even corrosion and to take preventive measures in the design than to take local corrosion attacks into account.
Bimetallic corrosion
- The size ratio between the cathode and the anode (a larger anode area compared to a cathode area reduces the galvanic effects)
- The magnitude of electrode potential difference
- Conductivity of the electrolyte (liquid)
Pit corrosion
Grain boundary corrosion
Intergranular corrosion occurs between the grain boundaries inside a metal. The grain boundaries act as anodes while the grains act as cathode surfaces. This type of corrosion is well known for stainless steels that are exposed for a long period of time at temperatures between 500 and 800 ° C. At this temperature, chromium will react with carbon at the grain boundaries and form carbides. This causes a deficiency of chromium near the grain boundaries. If the chromium content falls below 12%, corrosion can easily start.
Stress corrosion
Erosion corrosion. Erosion corrosion is a combination of electrochemical corrosion (ie general corrosion) and a turbulent flow of liquids. Attacks are generally localized to areas with turbulent flow and are promoted by gas bubbles and solid particles.
Filiform corrosion
Filiform corrosion occurs under painted or plated surfaces when moisture penetrates the coating. It is a type of localized corrosion that is often associated with aluminum and magnesium alloys that have an organic coating. Lacquered surface and quick-drying paints on a surface are most susceptible to the problem. Filiform corrosion tends to occur at high humidity, e.g. greater than 75% and temperatures above room temperature.

